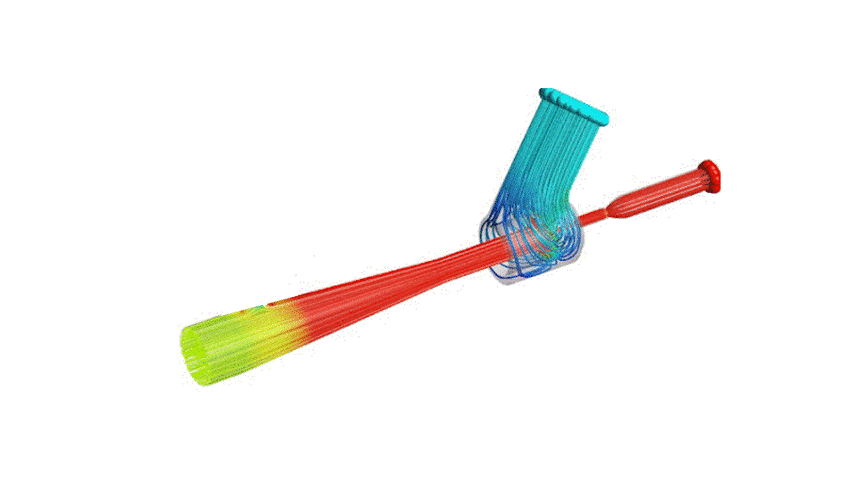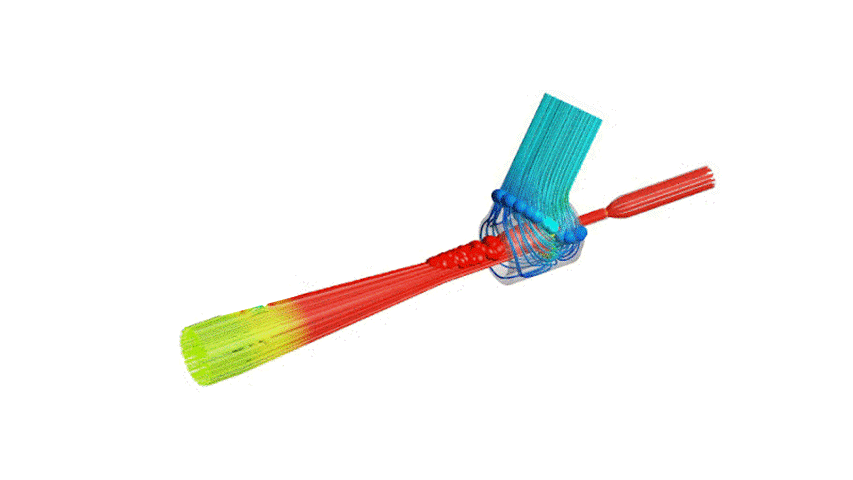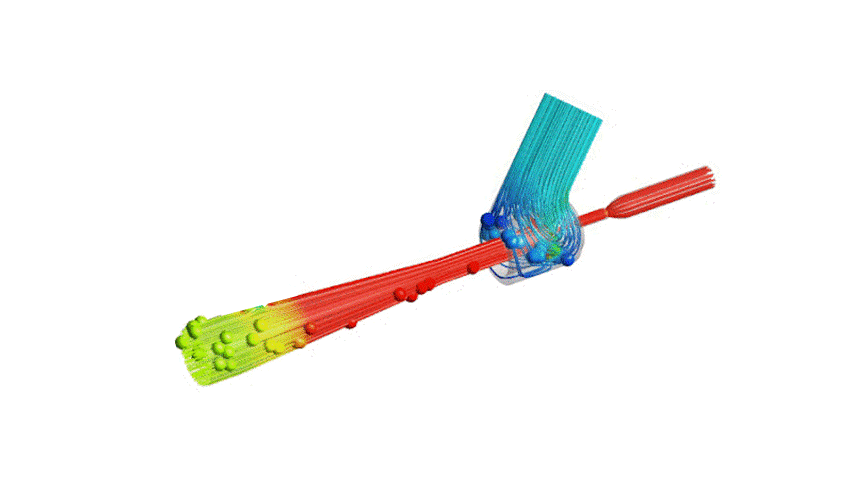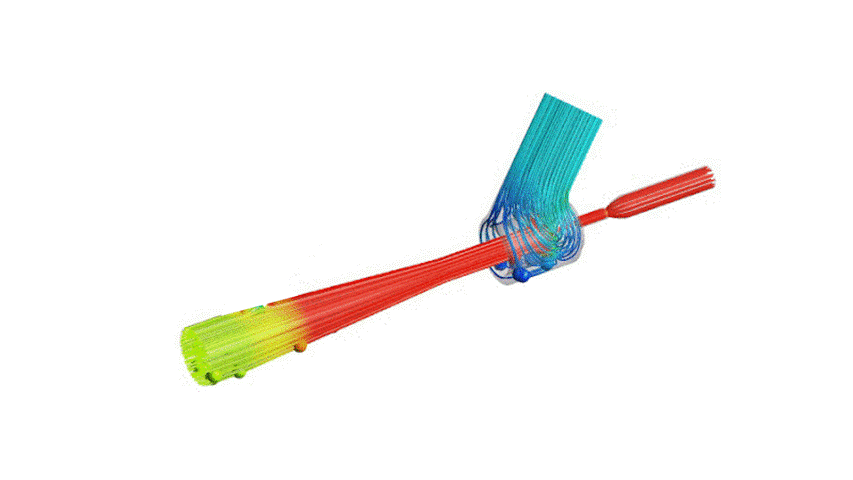
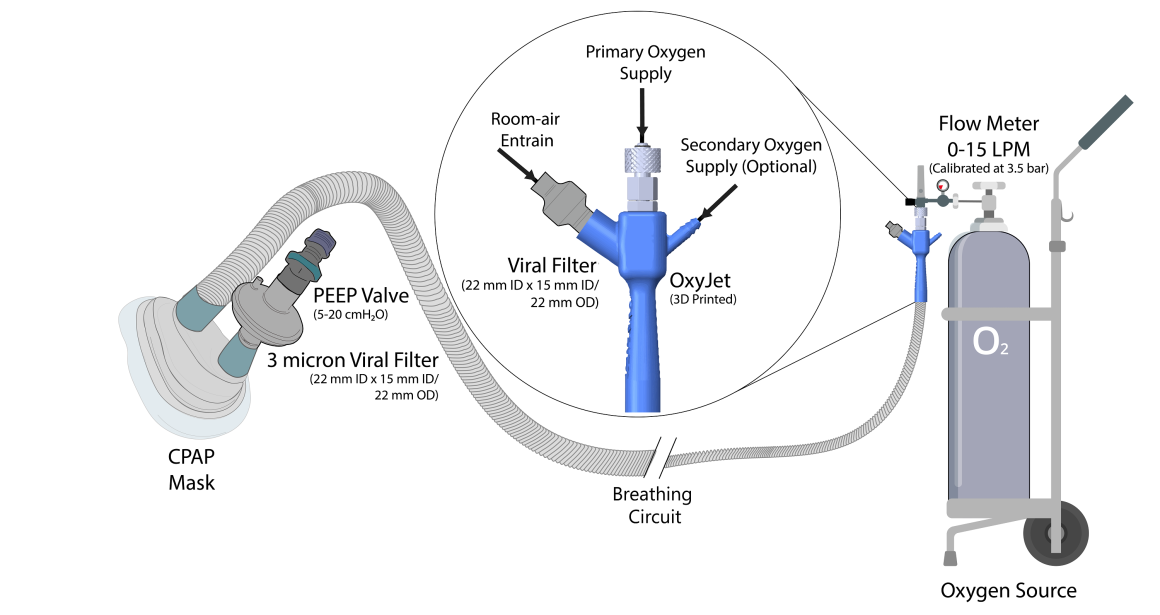
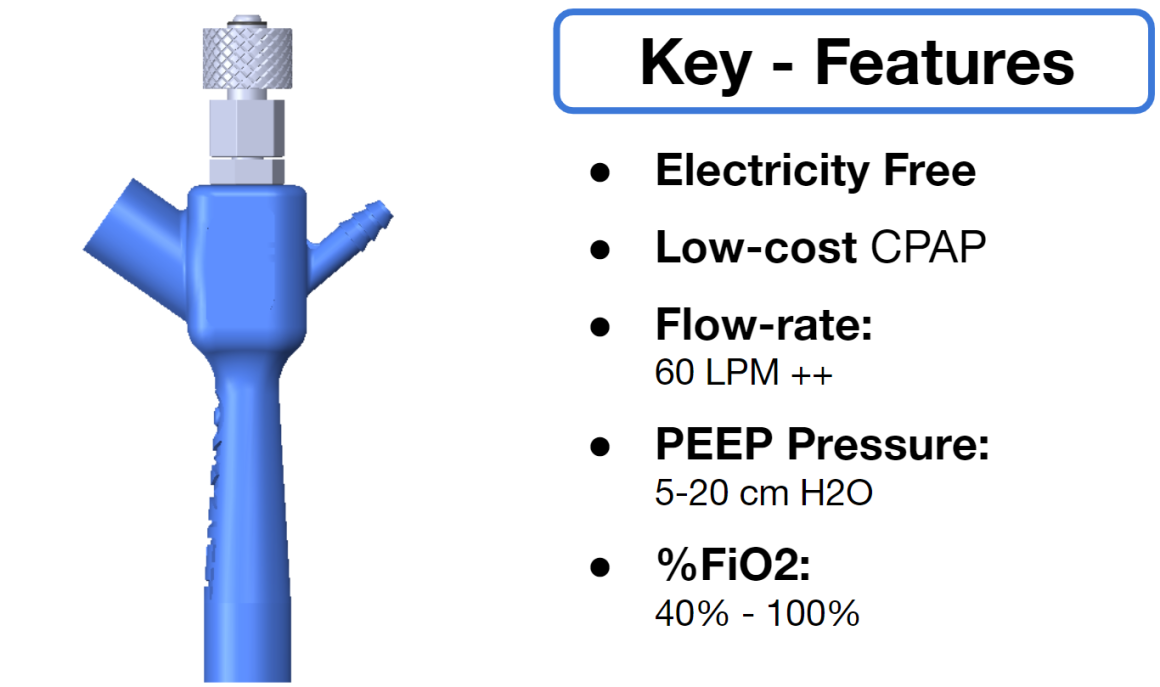
The Covid-19 pandemic has strained the hospital systems in many countries in the world, especially in the developing countries. In many low-resource hospitals, severely ill hypoxemic Covid-19 patients are treated with various forms of low-flow oxygen therapy (0-15 L/min), including interfaces such as, nasal cannula, hudson mask, venturi-mask, and non-rebreather masks. When the oxygen saturation of patients cannot be maintained on 15L/min of pure oxygen flow, international treatment guidelines suggest non-invasive positive pressure ventilation (NIPPV) or high-flow nasal oxygenation (HFNO) as the next stage of treatment to avoid invasive mechanical ventilation (IMV). However, administering high-flow nasal oxygenation (HFNO) in the general wards of a low-resource hospital is difficult due to a number of factors, including difficulty in operation, unavailability of electric power outlets and frequent maintenance. Therefore, in many cases the highest level of care a patient receives in the general ward is 15L/min of oxygen on a Non-Rebreather Mask. With shortage of Intensive Care Unit (ICU) beds, this is a major problem since intermediate forms of treatments are simply not available at an affordable cost. To address this gap, we have developed the OxyJet CPAP, a low-cost CPAP system specifically designed for low and middle-income country (LMIC) hospitals. The device is a precision venturi-based flow-generator capable of providing up to 60L/min of flow. The device utilizes the mechanics of a jet pump driven by high-pressure oxygen to increase the volumetric flow rate by entraining atmospheric air. Using a dual-flowmeter, the fraction of inspired oxygen (FiO2) can be attained between 40 – 100%. Consisting of a traditional 22mm breathing circuit, a non-vented CPAP mask, and a Positive End-Expiratory Pressure (PEEP) valve, the CPAP can provide positive pressures between 5-20 cm H2O. The device is manufactured using local 3D printing and workshop facilities. The pressure and flow characteristics of OxyJet are equivalent to existing CPAP devices and the technical evaluations meet the requirement of the UK-MHRA Rapidly Manufacturable CPAP systems (RMCPAPS) guideline.